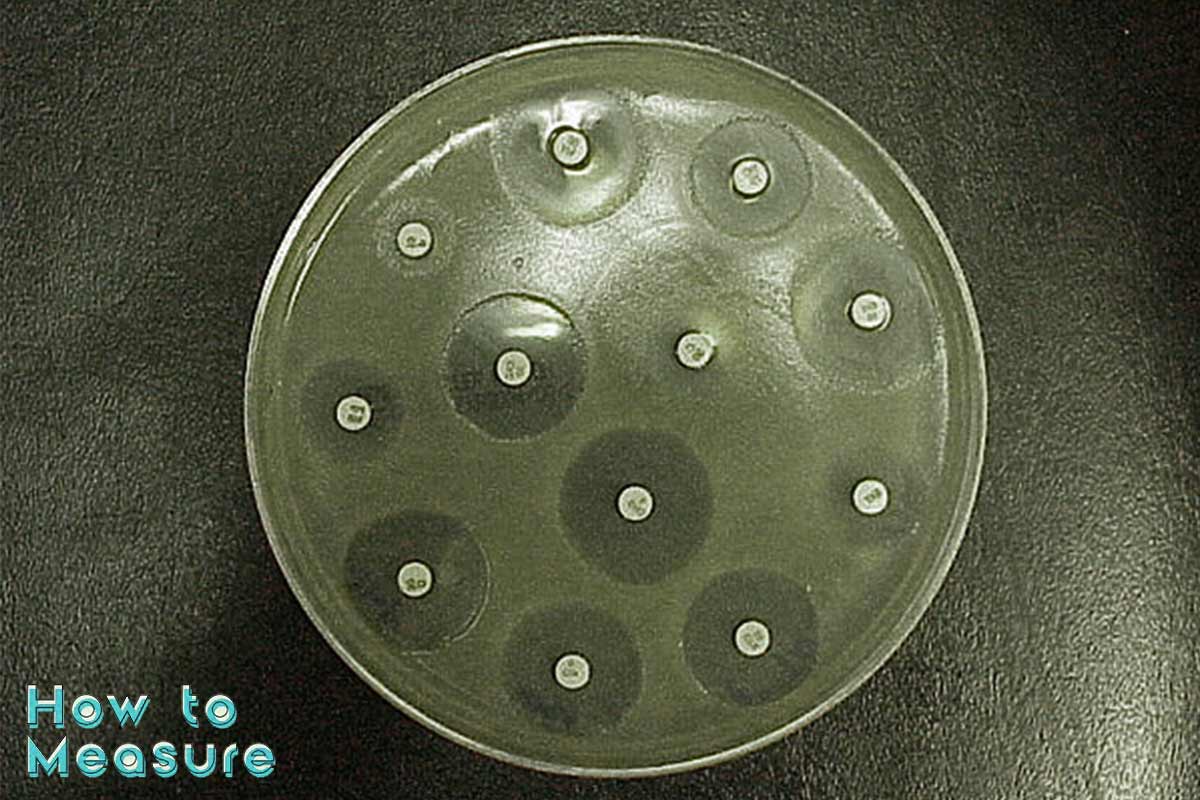
The Kirby-Bauer test is a method for measuring the sensitivity of bacteria to antibiotics. A paper disc impregnated with an antibiotic is placed on a plate containing bacterial cells, and the diameter of the zone of inhibition is measured after incubation at 37°C for 24 hours. The diameter of the zone of inhibition gives information about the potency of a drug as it relates to its ability to inhibit bacterial growth.
Kirby-Bauer Test
The Kirby-Bauer test determines the zone of inhibition of bacterial growth, which is expressed as a percentage. It’s used to determine whether or not antibiotics are effective against certain bacteria.
The procedure involves taking a sample from an agar plate and streaking it onto another agar plate containing the antibiotic being tested. The bacteria will grow in both areas: one where there is no antibiotic and one where there is an active concentration of antibiotic present. The area where no growth occurs (called “zone”) provides information about how well that particular drug works against that particular type of bacteria because if it has been inhibited from growing there, then we know that this particular drug would be effective in treating someone infected with these types of infections as well!
The Procedure of the Kirby-Bauer Test
Procedure:
- Prepare a culture of the bacterial strain to be tested by rehydrating a pure culture on agar plates and incubating them overnight at 37C.
- Prepare three sterile tubes containing 5ml of nutrient broth per tube: one tube will contain no antibiotic, one will contain an antibiotic; and one will contain both the bacterium and the antibiotic (positive control). The positive control is used to determine if there is any inhibition occurring within your experiment or whether it was due to contamination of your medium with other organisms in its preparation or growth mediums used for growing cultures.
- Inoculate each tube with 0.1ml from each culture plate using a sterile inoculating loop before adding 10ml of nutrient broth containing 100mg/L tetracycline (or other appropriate concentration)
Results of the Kirby-Bauer Test
The results of a Kirby-Bauer test are usually interpreted as follows:
- Positive: Growth of bacteria is inhibited around the edge of a hole punched through the plate by an antibiotic disc. This indicates that the bacterium is susceptible to that particular drug and may be a good candidate for treatment with that antibiotic.
- Negative: No growth occurs around holes punched through the plate by an antibiotic disc, indicating resistance to that drug.
Materials
- Agar plates (one per test)
- Tubes containing antibiotics (one per test)
- Sterile swabs/swatches (one per test)
- Sterile water, 90% ethanol, sterile pipette tips and tubes; be careful not to contaminate the agar media with bacteria or fungi.
Procedure
- Place the agar plates on a flat surface
- Take the inoculum and spread it evenly over the surface of the agar plates
- Let the plates sit for 24 hours under proper incubation conditions
- Measure the diameter of your zone of inhibition
Measure Zone of Inhibition Kirby-Bauer
To measure the zone of inhibition kirby-bauer, you will need:
- Sterile cotton swabs
- The aseptic technique (i.e., gloves)
- Transfer the inoculum from your tube into a sterile container using a sterile cotton swab
- Spread the inoculum across the surface of your agar with another sterile cotton swab
The Kirby-Bauer Test is a Method for Measuring the Sensitivity of Bacteria to Antibiotics
It is based on the ability of Escherichia coli to form colonies on agar plates containing different concentrations of antibiotic, which can then be counted by eye or using an automated colony counter.
The standard protocol calls for preparing two identical sets of 96-well plates (one as a control and one with an antibiotic), inoculating each well with an overnight culture, incubating them at 37 degrees Celsius (98 F) until they reach mid-exponential phase (~2 hours), then adding 0.5 microliters/mL of each concentration being tested before returning them to incubation at 37 degrees Celsius (98 F) overnight (18 hours). The next day you will see zones around where there was no growth because those areas are “immune” from the action of that particular antibiotic; these zones vary depending on how much you added, so if you want more accurate measurements, use less than what’s recommended above.”
Conclusion
The Kirby-Bauer test is a method for measuring the sensitivity of bacteria to antibiotics.