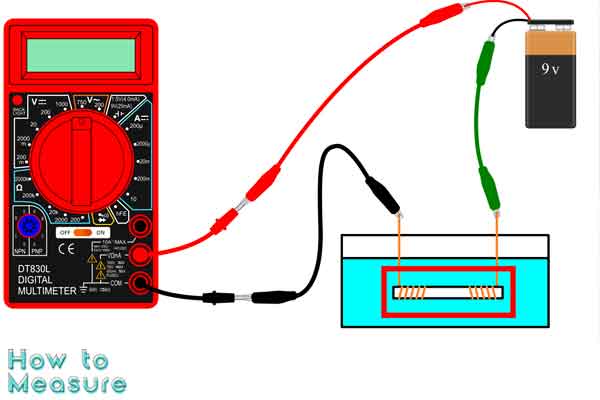
Electrolytes are essential components of many chemical and biological systems, and measuring their concentration accurately is important for understanding their behavior and effects. Multimeters are versatile tools that can be used for a wide range of electrical measurements, including the measurement of electrolytes. With the right equipment and techniques, it is possible to measure the concentration of electrolytes in a solution using a multimeter. This can be a useful tool for scientists, engineers, and researchers in chemistry, biology, and environmental science, where precise measurements of electrolyte concentrations are often necessary. This guide will provide an overview of how to measure electrolytes with a multimeter, including the necessary equipment, preparation, and measurement techniques.
What type of multimeter to use for measuring electrolytes?
When measuring electrolytes with a multimeter, it is important to use a meter capable of accurately measuring the electrical conductivity of a solution. For this reason, a digital multimeter with a conductivity measurement feature is recommended.
Two types of multimeters are commonly used for measuring electrolytes: handheld and benchtop multimeters. Handheld multimeters are portable and convenient for field measurements, while benchtop multimeters are more accurate and have more advanced features but are usually used in laboratory settings.
When selecting a multimeter for measuring electrolytes, it is important to ensure that it has a conductivity measurement mode with a suitable range. The conductivity range of the multimeter should be appropriate for the expected range of electrolyte concentrations in the solution being measured. Most multimeters have a range that can measure conductivity from micro Siemens (µS) to millisiemens (mS).
Another important factor to consider is the accuracy and precision of the multimeter. The multimeter should be capable of providing accurate and precise measurements of conductivity. In addition, the multimeter should have a high input impedance to minimize errors due to leakage currents.
When selecting a multimeter for measuring electrolytes, it is important to consider the expected range of electrolyte concentrations, the accuracy and precision of the meter, and the measurement environment (field or laboratory).
What are the necessary probes and equipment for measuring electrolytes with a multimeter?
To measure electrolytes with a multimeter, you will need certain probes and equipment, including:
Conductivity probe: The most important probe to measure electrolytes with a multimeter. The conductivity probe is usually a pair of electrodes immersed in the measured solution. The conductivity probe measures the electrical conductivity of the solution and converts it into a reading that can be displayed on the multimeter.
Calibration solutions: Calibration solutions are used to ensure the accuracy of the conductivity probe. These solutions are usually available in different concentrations and are used to calibrate the multimeter before taking measurements. Calibration solutions should be selected based on the expected range of electrolyte concentrations in the measured solution.
Standard solutions are solutions with a known electrolyte concentration being measured. These solutions are used to verify the multimeter measurements’ accuracy and create a calibration curve.
Glass beaker: A glass beaker holds the electrolyte solution being measured. It is important to use a clean beaker to avoid contamination of the solution.
Stirrer or magnetic stir bar: A stirrer or magnetic stir bar ensures the electrolyte solution is well-mixed and homogeneous. This is important to ensure accurate and consistent measurements.
Temperature probe: Some multimeters have built-in temperature sensors to compensate for temperature variations. If your multimeter does not have a built-in temperature sensor, a separate temperature probe may be needed to measure the solution’s temperature.
Safety equipment: It is important to wear safety equipment such as gloves, safety goggles, and lab coats when handling electrolytes. This will protect you from chemical exposure and prevent contamination of the solution.
These are the probes and equipment to measure electrolytes with a multimeter. It is important to carefully follow the manufacturer’s instructions and safety guidelines when using these tools to ensure accurate and safe measurements.

How to calibrate a multimeter for accurate electrolyte measurements?
To calibrate a multimeter for accurate electrolyte measurements, you will need to follow the manufacturer’s instructions and use the appropriate calibration solutions. Here are the general steps for calibrating a multimeter for accurate electrolyte measurements:
- Ensure that the multimeter is set to the correct conductivity measurement mode.
- Select the appropriate calibration solution based on the expected range of electrolyte concentrations in the measured solution.
- Immerse the conductivity probe in the calibration solution and wait for the multimeter to stabilize and display a reading.
- Adjust the calibration controls on the multimeter until the displayed reading matches the known value of the calibration solution.
- Repeat this process with other calibration solutions of different concentrations to create a calibration curve.
- Verify the multimeter’s accuracy by measuring a standard solution of known electrolyte concentration and comparing the reading with the expected value.
- If the multimeter readings are not accurate, adjust the calibration controls and repeat the calibration process until the multimeter provides accurate and consistent readings.
It is important to note that the calibration of a multimeter for electrolyte measurements should be performed regularly, especially if the meter is subjected to changes in temperature, humidity, or frequent use. Additionally, it is important to use high-quality calibration solutions to ensure accurate and reliable measurements.
What are the units of measurement for electrolytes in a solution, and how to convert the reading from the multimeter?
The units of measurement for electrolytes in a solution depend on the type of electrolyte being measured. Here are the common units of measurement for some of the most frequently measured electrolytes:
- Sodium (Na+) and potassium (K+): milliequivalents per liter (mEq/L) or millimoles per liter (mmol/L)
- Chloride (Cl-): milligrams per deciliter (mg/dL) or millimoles per liter (mmol/L)
- Calcium (Ca2+): milligrams per deciliter (mg/dL) or millimoles per liter (mmol/L)
- Magnesium (Mg2+): milligrams per deciliter (mg/dL) or millimoles per liter (mmol/L)
It would be best to use the conversion factor for the measured electrolyte to convert the reading from the multimeter to the appropriate units. The conversion factor can be found in reference materials or calculated using the molecular weight and valence of the electrolyte.
For example, to convert a multimeter reading of conductivity (in µS/cm or mS/cm) to milliequivalents per liter (mEq/L) of sodium chloride (NaCl), you would need to use the conversion factor of 58.44. The formula for the conversion is:
mEq/L = (conductivity in µS/cm or mS/cm) / (58.44 x concentration in g/L)
It is important to note that the conversion factors may vary depending on the specific electrolyte being measured and the units of measurement used by the multimeter. Therefore, using the appropriate conversion factors and reference materials is important to ensure accurate and reliable measurements.
How to prepare the electrolyte solution for measurement with a multimeter?
The preparation of the electrolyte solution for measurement with a multimeter depends on the specific electrolyte being measured and the purpose of the measurement. Here are the general steps for preparing an electrolyte solution for measurement with a multimeter:
- Select the appropriate electrolyte solution based on the measured electrolyte type and the concentration range of interest.
- Ensure that the solution is clean and free from any impurities or contaminants that may affect the accuracy of the measurement.
- Measure the desired amount of the electrolyte solution using a clean beaker or pipette.
- If necessary, adjust the pH of the solution to the desired range using a pH meter or indicator.
- Mix thoroughly using a stirrer or magnetic stir bar to distribute the electrolyte throughout the solution evenly.
- Measure the temperature of the solution using a thermometer and adjust the temperature compensation on the multimeter if necessary.
- Immerse the conductivity probe into the solution and wait for the reading to stabilize.
- Record the reading displayed on the multimeter and convert the reading to the appropriate units of measurement using the conversion factors for the specific electrolyte.
It is important to note that the preparation of the electrolyte solution may vary depending on the specific requirements of the experiment or application. Therefore, it is important to carefully follow the instructions and guidelines provided by the manufacturer or reference materials to ensure accurate and reliable measurements.
How do you troubleshoot common problems encountered while measuring electrolytes with a multimeter?
Here are some common problems that may be encountered while measuring electrolytes with a multimeter and some possible solutions to troubleshoot them:
- Unstable or fluctuating readings: This may be due to a dirty or damaged conductivity probe or a contaminated or poorly mixed solution. Clean the conductivity probe or replace it if necessary, and ensure the solution is well-mixed and impurities-free.
- Inaccurate readings: This may be due to incorrect calibration or temperature compensation settings or an inappropriate or expired calibration solution. Check the calibration and temperature compensation settings and recalibrate the multimeter if necessary. Ensure that the calibration solutions used are fresh and have not expired.
- High or low readings: This may be due to using an inappropriate range setting on the multimeter or measuring a different electrolyte or concentration than intended. Check the range setting on the multimeter and ensure that the correct electrolyte and concentration are being measured.
- No readings: This may be due to a dead battery, damaged or improperly connected probes, or a faulty multimeter. Replace the battery or probes if necessary, or troubleshoot or replace the multimeter.
- Interference from other electrical devices: This may cause noise or distortion in the readings. Move the multimeter and probes away from other electrical devices or sources of interference.
It is important to note that troubleshooting common problems encountered while measuring electrolytes with a multimeter requires careful attention to detail, patience, and a systematic approach. Carefully follow the manufacturer’s instructions and reference materials, and seek assistance from qualified professionals if necessary.
What are the safety precautions to take while measuring electrolytes with a multimeter?
Measuring electrolytes with a multimeter involves working with potentially hazardous chemicals and electrical equipment. Here are some safety precautions to take while measuring electrolytes with a multimeter:
- Wear appropriate personal protective equipment (PPE) such as gloves, safety goggles, and a lab coat to protect against chemical spills, splashes, and electrical shock.
- Ensure the work area is well-ventilated to minimize exposure to fumes or vapors.
- Follow proper handling and storage procedures for the electrolyte solutions, including labeling and storage in a secure and designated area.
- Ensure that the multimeter is in good working condition and properly grounded to minimize the risk of electrical shock or damage to the equipment.
- Always disconnect the multimeter from the power source before making any adjustments or maintenance.
- Avoid touching the conductivity probes immersed in the electrolyte solution to prevent contamination or electrical shock.
- Be aware of the potential hazards and risks associated with the specific electrolytes being measured, and take appropriate precautions to minimize exposure and risks.
- Follow the manufacturer’s instructions and guidelines for safely and properly using the multimeter and other equipment.
It is important to note that the safety precautions may vary depending on the specific electrolyte being measured, the concentration range of interest, and the specific equipment and procedures used. Therefore, it is important to carefully follow the instructions and guidelines provided by the manufacturer or reference materials and seek assistance from qualified professionals if necessary.
Conclusion
In conclusion, measuring electrolytes with a multimeter is important in many laboratory applications and industries. It requires appropriate equipment, such as a multimeter with conductivity probes, careful attention to detail, including proper calibration and temperature compensation, and preparing a clean and well-mixed electrolyte solution. The risk of hazards and risks can be minimized by following the appropriate safety precautions, such as wearing appropriate personal protective equipment and handling the electrolyte solutions with care. Troubleshooting common problems, such as inaccurate or unstable readings, can also be addressed by carefully following the manufacturer’s instructions and seeking assistance from qualified professionals when necessary. With proper care and attention, measuring electrolytes with a multimeter can provide accurate and reliable results for various applications.